1 July 2021. If you are a member of the public looking for information and advice about coronavirus COVID-19 including information about the COVID-19 vaccine go to the NHS website.
Murdoch Childrens Research Institute in Melbourne Australia is conducting a Phase III trial of the bacillus Calmette-Guérin BCG tuberculosis vaccine to see if it also protects against the coronavirus.
Which coronavirus vaccine is in phase 3. BNTX today announced that after conducting the final efficacy analysis in their ongoing Phase 3 study their mRNA-based COVID-19 vaccine candidate BNT162b2 met all of the studys primary efficacy endpoints. AstraZeneca COVID-19 vaccine Novavax COVID-19 vaccine. Has officially filed for a biologics license application BLA to the United States Food and Drug Administration FDA that would lead to full licensure.
Indias First mRNA Vaccine Safe Gets Nod for Phase 2 and 3 Trials. COVID-19 Vaccines Approved for Emergency Use or in Phase 3 Trials. TOKYO -- Daiichi Sankyo plans to begin Phase 3 clinical trials for its coronavirus vaccine this autumn with the aim of bringing it to the public in 2022 finally pushing forward Japans effort to.
COVID-19 Vaccines Approved for Emergency Use. Vaccine trials usually undergo three rounds of testing. Nat Med 27 205211 2021.
The Pune-based biotechnology company had submitted the interim clinical data of the vaccines Phase 1 study to the. These COVID-19 vaccine candidates are in phase 3 clinical trials but that does not mean that any or all will be safe and effective. The US has a fourth of global.
With data from its Phase 3 COVE study showing a COVID-19 vaccine with 93 percent effectiveness Moderna Inc. Seven COVID-19 vaccines in the third phase of clinical trials. The trial called BRACE is being run in Australia Brazil the Netherlands Spain and the United Kingdom.
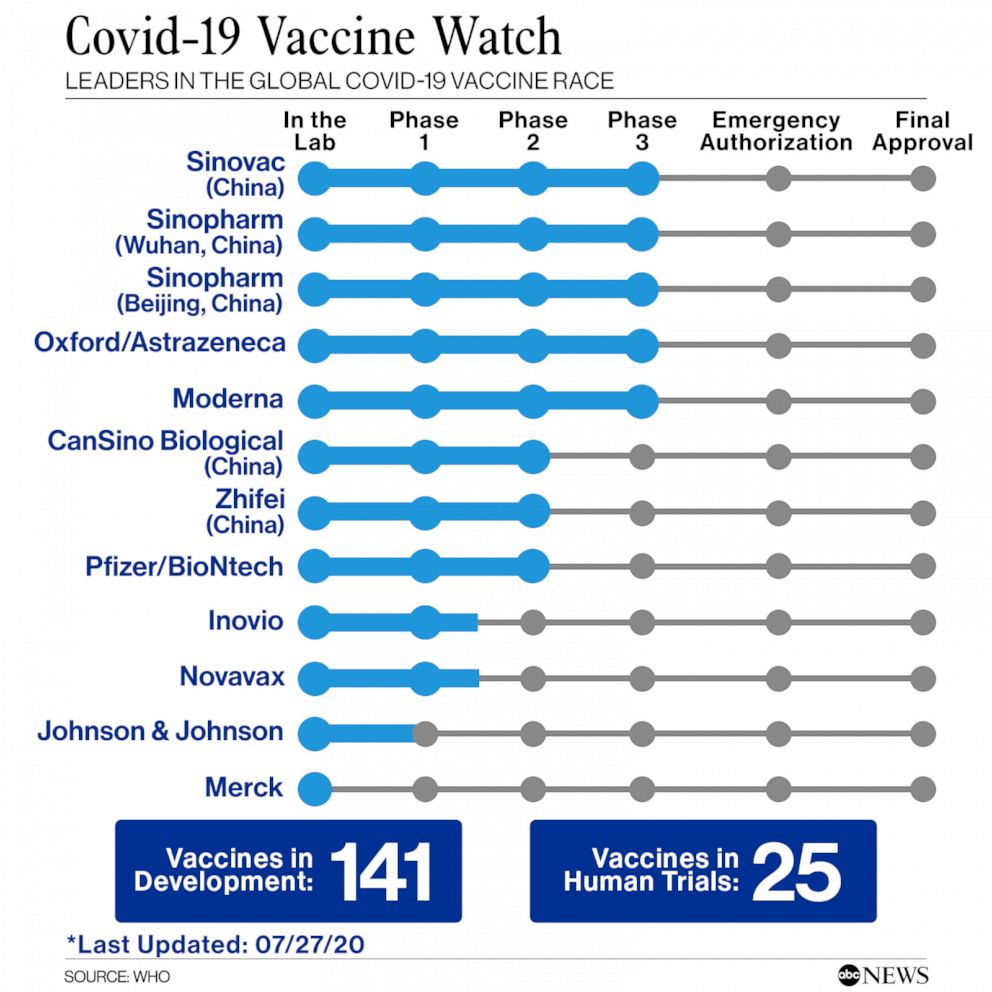
While close to 200 vaccines are being developed for COVID-19 at least seven candidates have now moved to the phase 3 human trials - the final stage of testing to prove whether the vaccine is safe and effective against the virus before getting FDA approval. Our advice for clinicians on the coronavirus is here. Savannah Georgia CNN The first Phase 3 clinical trial of a coronavirus vaccine in the United States began Monday.
Kim JH Marks F. A Phase 3 clinical trial designed to evaluate if an investigational vaccine can prevent symptomatic coronavirus disease 2019 COVID-19 in adults has begun. Basic principles of COVID-19 vaccination autumnwinter phase 3 which are designed to support your planning locally.
The mRNA vaccine is developed in partnership with Department of Biotechnology and. New Delhi Aug 24 PTI Indias first mRNA-based COVID-19 vaccine candidate HGCO19 by Gennova Biopharmaceuticals Ltd has been granted approval for Phase 23 clinical trails the Department of Biotechnology DBT said on Tuesday. You can also find guidance and support on the GOVUK website.
Phase 1 phase 2 and phase 3. Over 140 candidate vaccines have been developed globally and 18 of them are currently in human trials. Looking beyond COVID-19 vaccine phase 3 trials.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus that causes coronavirus disease 2019 Prior to the COVID19 pandemic an established body of knowledge existed about the structure and function of coronaviruses causing diseases like severe acute respiratory syndrome SARS and Middle. Thats the point of phase 3 clinical trials. The table belows shows COVID-19 vaccines that have been approved for emergency use green have reported vaccine efficacy from a Phase 3 trial blue or are under investigation in a Phase 3 trial.
Vaccines in Phase 3 Clinical Trials As of February 27 2021 large-scale Phase 3 clinical trials are in progress or being planned for two COVID-19 vaccines in the United States. In my previous post I suggested that while were pursuing Phase 3 testing of several promising Covid-19 vaccines we could simultaneously offer those same unapproved vaccines.
The Moderna and Pfizer-BioNTech vaccines offer immunity against COVID-19 for at least six months and might offer protection for up to two to three years. The CEO of one vaccine maker said immunity may start to fade within a year.
Covid Vaccine Pfizer Says 94 Effective In Over 65s Bbc News
With the two-shot vaccines people are more likely to report.

How long does pfizer coronavirus vaccine last. The fact that the reactions continued for almost four months after vaccination thats a very very good sign Dr. The clinical trials performed on the Pfizer vaccine show its approximately 95 effective against symptomatic COVID-19 around seven days after receiving two doses. Vaccination helps protect you even if youve already had COVID-19.
In April a report in The New England Journal of Medicine NEJM said that in all 33 participants who had received the Moderna vaccine during the Phase I trial protection remained high for six months after the second shot. The unpublished study data follows news that its two-dose mRNA vaccine may be less effective at preventing the spread of COVID-19 over time particularly at 6 months after vaccination. It means Pfizers shot for people 16 and older has now undergone the same rigorous testing and regulatory review as dozens of other long-established vaccines.
Research has shown that immunity following natural infection remains for at least eight months and it may be even longer. Trial data for the Pfizer-BioNTech vaccine has revealed that even after 85 days of the second vaccination dose the body still had the necessary antibodies to protect against SARS CoV-2. However they will most likely have to be administered annually.
We dont yet know how long youll be protected or how much it stops you from catching and passing on the virus. It means Pfizers shot for people 16 and older has now undergone the same rigorous testing and regulatory review as dozens of other long-established vaccines. One of the most pressing questions about COVID-19 vaccines is how long they can provide protection.
Pfizer and BioNTech announced Thursday that the Pfizer COVID-19 vaccine was 913 effective at stopping symptomatic COVID-19 for at least six months. The latest report from vaccine maker Pfizer shows people in South Africa who got its coronavirus vaccine after B1351 became the dominant circulating virus were still. That same month Pfizer reported that its vaccine was still highly effective at six months.
In April both Pfizer and Moderna reported that their vaccines provided at least six months of protection. This is the latest. According to a recent announcement made by Pfizer on April 1 its vaccine offers six months of strong protection against symptomatic COVID-19.
Last month coincidentally on April Fools Day Pfizer and BioNTech announced that among their Phase 3 clinical trial participants the Pfizer-BioNTech Covid-19 vaccine affectionately known as. How safe is the Pfizer vaccine. Germinal centers typically peak one to two weeks after.
New research from Pfizer found a drop in protection after six months although the vaccine remained highly effective against severe illness. The effectiveness of Pfizers COVID-19 shot can drop to 837 within four to six months after getting the second dose of its vaccine. Studies of two of the most prominent COVID-19 vaccines suggest they remain effective for at least six months.
How long does the Pfizer Vaccine last. Evidence is emerging that people get better protection by. The organisations phase 3 trial revealed that the Pfizer vaccine was 913 effective at preventing COVID-19 for up to six months after the second dose and 100 effective against severe disease as defined by.
Their reports were based on tracking whether vaccinated people. The Pfizer-BioNTech novel coronavirus vaccine was the first to get approved for emergency use in the UK followed by the US and other countries around the world. Research has not yet shown how long you are protected from getting COVID-19 again after you recover from COVID-19.
According to the CDC side effects usually start within a day or two of getting the vaccine but they should also go away in a few days Are side effects more likely after the first or second dose. The ongoing study which has yet to be peer-reviewed shows that the PfizerBioNTech vaccine was overall 91 percent effective at preventing COVID-19 infection over the course of six months.
And South America provides firmer answers to lingering questions about the vaccines efficacy and whether it protects older adults equally well. AstraZeneca and the University of Oxford reported that preliminary results from studies being conducted in the UK.

First Peer Reviewed Results Of Phase 3 Human Trials Of Oxford Coronavirus Vaccine Demonstrate Efficacy University Of Oxford
But in order to do so the company needs to.
Astrazeneca coronavirus vaccine phase 3 results. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use. Another vaccine option in coming months. UPDATED ON OCT 14 2020 0225 PM IST The results of phase three of Oxford-AstraZeneca Covid-19 vaccine could be available by November-end or early.
The result which comes from a Phase 3 trial of 32449 participants in the US. First peer-reviewed results of phase 3 human trials of Oxford coronavirus vaccine demonstrate efficacy University of Oxford. But in order to do so the company needs to smooth.
Results from the subgroup comparisons presented in this analysis were similar to overall results table 3. These results together with the induction of both humoral and cellular immune responses support large-scale evaluation of this candidate vaccine in an ongoing phase 3. AstraZeneca released Phase 3 data showing promising results for a combination antibody therapy that prevents COVID-19 possibly opening the door.
Phase 3 AstraZeneca Coronavirus Vaccine Study Reopens at URMC Update November 23 2020. CNN British drugmaker AstraZeneca said Monday it has started Phase 3 trials of its experimental coronavirus vaccine in the United States becoming. Late-stage or Phase III trials are ongoing to confirm the findings researchers said and to test whether the vaccine protects against infection with SARS-CoV-2 in a broad range of people.
We do not know if COVID-19 Vaccine AstraZeneca is as effective in people with immunocompromise compared with the rest of the population. AstraZeneca today announced interim results of their phase 3 US study which indicate 79 overall efficacy of their vaccine against symptomatic COVID. AstraZeneca AZN anticipates having Phase 3 trial results by the end of March potentially giving the US.
A Phase III Randomized Double-blind Placebo-controlled Multicenter Study in Adults to Determine the Safety Efficacy and Immunogenicity of AZD1222 a Non-replicating ChAdOx1 Vector Vaccine for the Prevention of COVID-19. Actual Primary Completion Date. AstraZeneca AZN anticipates having Phase 3 trial results by the end of March potentially giving the US.
Another vaccine option in coming months. In The Lancet Merryn Voysey and colleagues1 report the updated primary efficacy results for the OxfordAstraZeneca ChAdOx1 nCoV-19 AZD1222 vaccine from three single-blind randomised controlled trials in the UK and Brazil and one double-blind study in South Africa24 The ChAdOx1 nCoV-19 vaccine was granted emergency use authorisation in adults by the UK Medicines and Healthcare. In the SDSD UK cohort who were aged 1855 years 49 cases were available for inclusion in the analysis and vaccine efficacy was 593 95 CI 251 to 779.
Adult participants in the phase 2 and 3 trials will be randomized to receive one or two doses of AZD1222 or a vaccine against meningococcal bacteria that will serve as the control. Actual Study Start Date. A clinical trial is being conducted of COVID-19 Vaccine AstraZeneca given to people with stable HIV infection with results expected in a few months.
And Brazil show that the vaccine is up to 90 percent effective depending upon the dosing regimen. But in order to do so the company needs to. AstraZeneca anticipates having Phase 3 trial results by the end of March potentially giving the US.
Another vaccine option in coming months. Oxford and AstraZeneca researchers present a pooled analysis in The Lancet of Phase 3 trials of a coronavirus vaccine resulting in an average efficacy of 704. AstraZeneca got very encouraging results from the Phase 3 Provent study for a new mix of antibodies monoclonal which could replace the Covid vaccineIts about a pre-exposure prophylaxis similar to the PrEP that is prescribed today to patients at risk of contracting HIV and which prevents contagion from Covid-19LAzd7442 this is its production name has proved effective in reducing the.
Estimated Study Completion Date.